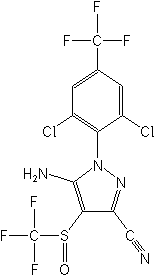
- Basic data
There are two main types of toxicities for any substance: acute toxicity, which corresponds to the absorption of a massive dose of toxicant in a single dose, or more rarely several doses over a period of 24 hours. maximum, and the chronic toxicity which corresponds to the absorption of low or very low doses of toxic repeated over a period ranging from several months to several years.
The acute toxicity is measured by the lethal dose 50 or LD 50 which corresponds to the dose likely to kill 50% of the animals to which it is administered. This expression of toxicity is explained by the fact that not all individuals of the same species have exactly the same sensitivity for a toxic substance and that this sensitivity is distributed according to a log-normal distribution (ie the LD 50measure the average sensitivity of the species to the toxic). When the toxicant is present in the atmosphere , or water for aquatic animals, using lethal concentration 50 (LC 50 ) , a concentration which, for a duration given exposure, causes 50% of deaths. These measurements are made for various routes of entry of toxins and for at least two different animal species.
- LD 50 , oral (rat): 97 mg / kg
- LD 50 , oral (rabbit): 43 mg / kg
- Dl 50 dermal (rat): 2000 mg / kg
- Dl 50 dermal (rabbit): 354 mg / kg
- LC 50 respiratory tract (rat): 0.68 mg / l then 0.36 mg / l in 2003
The LC 50 are given per liter of air and for 4 hours of exposure. The LD 50 are expressed in mg of toxic per kg of body weight , which makes it possible to overcome the difference existing between the weight of a rat and that of a man.
Values can be slightly different depending on the date and the origin of the documents. It should be noted, for example, that COMTOX retains an LC 50 of 0.2 mg / l for Regent TS whereas it contains only 50% of Fipronil. This lower value seems paradoxical. It is not currently possible to know if it is due to additives present in the Regent that would allow a better absorption of Fipronil, or if it is a better evaluation of the real toxicity which would have been under- estimated in the first studies on pure Fipronil.
The acute toxicity of the product has been classified as moderate by the WHO and various other organisms, but we will see that this classification is a problem. The values quoted above make it possible to note that the respiratory route is very efficient , that the dermal route is not a major route, but that it shows a significant difference between the two species studied: negligible in the rat, it corresponds at moderate toxicity in rabbits. Dermal penetration in humans appears to be comparable to that of rats (based on poorly described experiences in a FAO document), but it is obvious that this penetration will be largely influenced by the form in which the Fipronil comes into contact with the skin (powder, solution in various solvents , the latter having a much higher risk).
The discussion around these values is very important because they determine the classification of substances as Very Toxic (T +), Toxic (T) or Harmful (Xn) and consequently the means of protection to be implemented. The regulatory labeling of chemicals and preparations includes, in addition to suggestive pictograms, risk phrases (R-phrases) and safety-related phrases (S-phrases) .
The latest assessments lead on these bases to the following classification for Fipronil:
- Toxic if swallowed (R25)
- Toxic in contact with the skin, taking into account the results for the rabbit (R24)
- Toxic by inhalation based on the value 0.36 mg / l. Very toxic by inhalation, taking into account the result for Régent TS (R26).
The various insecticides containing Fipronil may have different classifications since they contain a variable percentage of active substance .
For example the Regent TS must be classified:
- Harmful if swallowed (for single ingestion)
- Toxic, danger of serious damage to health by prolonged exposure if swallowed
- Very toxic by inhalation
This entails, by implication, the obligation to include on the label the phrases:
- Wear appropriate protective clothing (S36)
- Wear suitable gloves (S37)
- In case of insufficient ventilation , wear suitable respiratory equipment (S38)
For example, in a plant producing veterinary products, operators handling large quantities of pure Fipronil must wear a breathing apparatus . In spite of the risk of residual exposure when they subsequently remove the suit, it seems that it is not these operators who have the highest traces of Fipronil in the blood, but those who work on the final product packaging lines, where these are present as a dilute solution. This therefore suggests that the solvent facilitates penetration.
It is much more difficult to simply quantify chronic toxicity because of the potentially highly variable exposure patterns. An important criterion isthe no observable effect level (designated by the NOELs , no observable effect level or NOAEL , no observable adverse effect level). Depending on the modes of administration and the symptoms sought, one finds very different values. For the most demanding criteria a value of 0.02 mg / kg / day has been retained by the digestive tract.
- Other effects of Fipronil
These are effects that are not related to action on GABA receptors . Fipronil is known to disrupt hypothalamic-thyroid regulation : the concentration of thyroxine falls while TSH increases. At high doses, of the order of 15 mg / kg / day prolonged administration, adenomas and thyroid carcinomashave been observed in the rat (only this species). These observations suggest that Fipronil is not a carcinogendirect, but that cancerization is the indirect result of severe disruption of hypothalamic-thyroid regulation. Indeed the product gives negative results in all the other tests of demonstration of the carcinogenic power.
Cancerization therefore seems possible only if a high threshold of exposure is reached , which is different from true initiating carcinogens for which the probability of developing cancer decreases, with the dose, but becomes zero for zero exposure. On the other hand, the documents consulted unjustifiably claim that the cancer of the thyroid observed in the rat does not concern humans. However, the US Environmental Protection Agency (EPA) classifies the product as possibly carcinogenic to humans (Group C, which is the lowest level).
- Solubility of Fipronil
Fipronil is extremely insoluble in water. It is then conventional in toxicology to evaluate the lipophilicity of this type of product, that is to say its ability to dissolve in fats. Indeed lipophilicproducts have a strong tendency (especially if they are only slowly degradable) to accumulate in adipose tissue and other organs rich in fat .
The method used is to measure the n-octanol / water partition coefficient . Some explanations are necessary to understand the continuation. Equal volumes of water and n-octanol (a fatty alcohol , thus immiscible with water) and a small amount of the test substance are placed in a container . The concentration of the substance is agitated and measured in each of the two phases. The partition coefficient is log (concentration in n-octanol / concentration in water) . For Fipronil this coefficient is 4, which means that this substance is 10,000 times more concentrated in n-octanol than in water. This value indicates that Fipronil is highly lipophilic, which implies a risk of significant concentration in adipose tissue or milk lipids .
This result, however, deserves to be discussed a little, which is not done in any document consulted. Indeed, various measurements show that Fipronil has a maximum solubility in short-chain carbon-containing solvents with an oxygen function (acetone, ethyl acetate, methanol) and a low or very low solubility in apolar or slightly polar solvents (0.028 g / l for hexane). Its solubility is also three times lower in n-octanol (8 carbons ) than in 2-propanol (3 carbons). However, lipids have longer carbon chains (typically up to 16-18 carbons). It is therefore clear that the actual lipophilicity of Fipronil is significantly lower than the n-octanol / water partition coefficient suggests.
This point is of some importance with regard to the risks of storage in the body and those related to the consumption of milk and milk products. However, the problem is too restrictive because Fipronil is metabolized fairly quickly in the body. What is found essentially in the tissues, and importantly indeed in the adipose tissue , is an accumulation of various metabolites whose toxicity is not negligible. Their partition coefficient does not seem to have been studied and the problem of the possible accumulation of Fipronil and its metabolites, as well as their passage in the milk, deserves further studies than those I found .