
By cleverly modifying the workings of photosynthesis by bacteria, researchers have managed to make them make hydrogen gas. What transform these microorganisms into plants to make biofuel used in fuel cells.
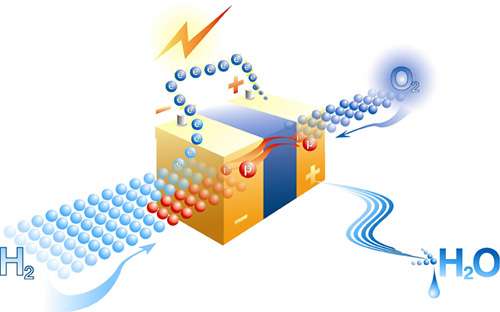
In order to produce the dihydrohene (H 2 ) that can be used in fuel cells that generate electricity and promise many uses, such as the electric car, scientists are exploring every possible path. Here is a new biochemists’ suggestion of diverting photosynthesis by bacteria.
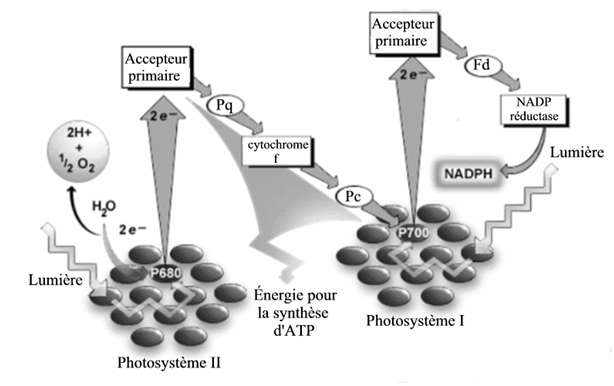
Used by plants, cyanobacteria and photosynthetic bacteria, photosynthesis produces energy (in the form of carbohydrates) from water, CO 2 and sunlight energy. To understand the idea of these scientists, we have to look a little at the inner workings of this biochemical mechanism. Schematically, molecular complexes called photosystems 1 and 2 capture photons and use the energy generated to release electrons (e – ). These negatively charged and highly energetic particles are in turn intercepted by different molecules including ferredoxins (“fd” in the diagram below). These then transfer the electrons to NADP reductase (or FNR, Ferredoxin: NADP +reductase), the enzyme responsible for storing the energy produced until it is used for carbohydrate synthesis.
It was at this stage of photosynthesis that the team led by Carolyn Lubner of Penn State University, whose results have just been published in the journal Pnas, became interested. These researchers have replaced the electron acceptor of the FNR, in this case NADP reductase, a hydrogenase, an enzyme that converts ions H + (one atom of hydrogen free electron) H 2 , c i.e., dihydrogen. These experiments were conducted on cyanobacteria called Synechococcus and bacteria belonging to the
After this biochemical DIY, the light causes in these microorganisms a release of electrons, which are then routed to hydrogenases associating them with H + ions to create dihydrogen. The result of these reactions is therefore a biofuel that can be used in fuel cells and is made from sunlight.
Is this biofuel profitable?
The researchers did not stop there. The same enzyme was used to join the metallic elements of all the photosystems 1 (on the right in the diagram above) of each bacterium. This molecular bond makes it possible to accelerate the transfer of the electrons and thus to increase the yield of the reaction. The system is even more effective if it is fed with vitamin C whose role is to provide enough electrons.
In the end, the researchers created a nano machinery allowing the production of hydrogen at a high rate. Indeed, the yield of modified photosynthesis is more than double what is observed naturally in cells. In addition, reactions creating hydrogen are able to function for several hours without depletion, as long as there is enough vitamin C.
The principle would be adaptable to other enzymes and other bacteria. We could then use these manipulations to produce different fuels.